EPOS/EUFOREA Update on Indication and Evaluation of Biologics in CRSwNP 2023
Biologics in Chronic Rhinosinusitis with Nasal Polyps
Co-Authors: Prof. Wytske Fokkens, Dr An-Sofie Visken, Prof. Vibeke Backer, Dr Diego Conti, Mrs Elizabeth Van Staeyen, Prof. Eugenio de Corso, Prof. Philippe Gevaert, Prof. Glenis Scadding, Prof. Martin Wagemann, Prof. Manuel Bernal-Sprekelsen, Dr Adam Chaker, Prof. Claire Hopkins, Prof. Enrico Heffler, Prof. Joseph K. Han, Prof. Joaquim Mullol, Prof. Peter Hellings, Prof. Anju Peters, Prof. Brent Senior
Severe chronic rhinosinusitis with nasal polyps (CRSwNP) is a debilitating disease with a significant impact on the quality of life (QoL). It is typically characterized by a type 2 inflammatory reaction and comorbidities such as asthma, allergies and NSAID-Exacerbated Respiratory Disease (N-ERD).
EUFOREA presents the new EPOS/EUFOREA update on the indication and evaluation of Biologics in Chronic Rhinosinusitis with Nasal Polyps 2023, discussing the practical guidelines for patients on biological treatment. In this newly published paper, the criteria for the selection of patients who would benefit from biologics were updated. The guidelines are proposed concerning the monitoring of the drug effects that provide recognition of responders to the therapy and, subsequently, the decision about continuation, switching or discontinuation of a biologic.
Figure 1: Adjustments in EPOS/EUFOREA criteria. The only change is the reduction of blood eosinophils to 150 cells/µl

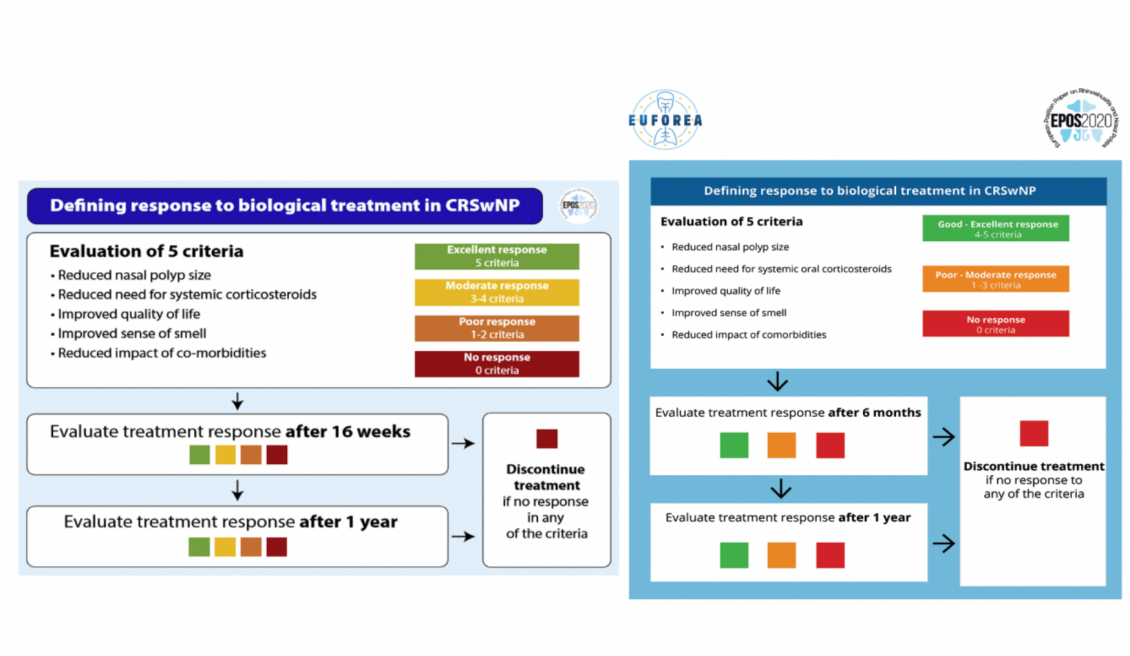
Figure 2: Adjustments in EPOS/EUFOREA response criteria